-
PDF
- Split View
-
Views
-
Cite
Cite
Lisa M. Sweeney, Melvin E. Andersen, Michael L. Gargas, Ethyl Acrylate Risk Assessment with a Hybrid Computational Fluid Dynamics and Physiologically Based Nasal Dosimetry Model, Toxicological Sciences, Volume 79, Issue 2, June 2004, Pages 394–403, https://doi.org/10.1093/toxsci/kfh116
Close - Share Icon Share
Abstract
Cytotoxicity in the nasal epithelium is frequently observed in rodents exposed to volatile organic acids and esters by inhalation. An interspecies, hybrid computational fluid dynamics and physiologically based pharmacokinetic (CFD-PBPK) dosimetry model for inhaled ethyl acrylate (EA) is available for estimating internal dose measures for EA, its metabolite acrylic acid (AA), and EA-mediated reductions in tissue glutathione (GSH). Nasal tissue concentrations of AA were previously used as the dose metric for a chronic Reference Concentration (RfC) calculation with this compound. However, EA was more toxic than expected, based on calculated tissue AA concentrations. Unlike AA, EA causes depletion of tissue GSH. We have developed an RfC for EA using tissue GSH depletion in the olfactory epithelium as the primary measure of nasal tissue dose. The hybrid CFD-PBPK model was refined to improve the accuracy of simulations for GSH in rat olfactory tissues. This refined model was used to determine the concentration for continuous human exposures to EA predicted to reduce nasal GSH levels to the same extent as seen in rats exposed to EA at the no-observed-effect level (NOEL). Importantly, AA concentrations in the human nasal olfactory epithelium at the proposed chronic RfC were predicted to be lower than the AA concentrations estimated in the rat at the NOEL. Thus, a chronic RfC based on maintaining GSH in the human nasal olfactory epithelium at levels equivalent to the rat NOEL would also provide an adequate margin of safety with respect to AA concentrations in nasal tissues.
Ethyl acrylate (EA; CAS No. 140–88–5) is a colorless liquid used as an intermediate in production of plastic resins for industrial and consumer use. Ethyl acrylate induced forestomach tumors in rodents administered high doses by gavage (National Toxicology Program, 1986) and was included in a list of compounds known or reasonably expected to be human carcinogens. However, in the Tenth Report on Carcinogens (National Toxicology Program, 2002), ethyl acrylate was removed from this listing. The decision to delist EA was based on the lack of tumorigenicity by other routes, evidence linking cancer in the gavage studies with site- and concentration-dependent irritation, and the unlikelihood of significant human exposure. Thus, concerns regarding the chronic toxicity of ethyl acrylate to humans pertain to noncancer effects. This work identifies an appropriate Reference Concentration (RfC) for chronic noncancer effects of ethyl acrylate for use in risk assessments for potentially exposed populations.
Similar to findings with other esters and volatile organic acids (Bogdanffy et al., 1987; Mainwaring et al., 2001; Miller et al., 1981), inhaled ethyl acrylate damaged the rodent nasal olfactory epithelium in acute and chronic studies (Frederick et al., 2002; Miller et al., 1985). In the Miller et al. (1985) study, mice and rats were exposed to ethyl acrylate for 6 h/day, 5 days/week for 27 months. This study established a no-observed-effect level (NOEL) of 5 ppm for nonneoplastic effects and a lowest observed effect level (LOEL) of 25 ppm for olfactory tissue damage in both rats and mice. Other effects, such as irritancy at the beginning of exposure, lethargy later in exposure, and decreased body weight gains, occurred at higher ethyl acrylate concentrations (Miller et al., 1985). Using nasal explants, Frederick et al. (2002) have demonstrated that olfactory lesions similar to those produced by in vivo exposure to ethyl acrylate may be produced by in vitro exposure to ethyl acrylate. These lesions are not produced in the presence of the esterase inhibitor, paraoxon. Furthermore, these lesions are similar to those produced in vivo and in vitro by acrylic acid (AA) (Frederick et al., 1998). Similarly, the concentration of an acid metabolite has been related to nasal and reproductive effects of other rapidly metabolized compounds (Bogdanffy et al., 1987; Sweeney et al., 2001; Welsch et al., 1995). These findings appear to support a mode of action for inhaled ethyl acrylate related to the concentration of AA in the olfactory epithelium.
The chronic inflammation associated with exposure to AA and its esters is believed to be the result of induction of the mitochondrial permeability transition (MPT) by AA. This chronic inflammation ultimately leads to the nonneoplastic lesions localized to the olfactory epithelium (Custodio et al., 1998). In the case of ethyl acrylate, esterase activity (leading to AA production) appears to be necessary to produce damage to the epithelium, since no damage was observed when rat nasal explants were incubated with high concentrations of ethyl acrylate (up to 1 mM) in the presence of the esterase inhibitor, paraoxon (Frederick et al., 2002). Depletion of hepatic GSH and other forms of oxidative stress enhance mitochondrial permeability (Haouzi et al., 2001; Hoek et al., 2002). A single exposure of rats to ethyl acrylate at the LOEL for olfactory damage, 25 ppm for 6 h, depleted olfactory epithelial GSH to 35–58% of the level in controls (Frederick et al., 2002). While AA appears to be required for nasal toxicity of ethyl acrylate, the depletion of GSH may enhance it. Sustained depletion of GSH was critical for the development of forestomach tumors in rats administered ethyl acrylate by gavage (Frederick et al., 1992). GSH depletion has been implicated in acute lethality and in biochemical effects of ethyl acrylate (Frederick et al., 2002; Vodicka et al., 1990).
The chronic toxicity studies for inhaled ethyl acrylate in rats (Miller et al., 1985) can form the basis for developing a safe exposure level for humans. While these interspecies extrapolations can be done using default procedures, the availability of the mode of action information and a computational fluid dynamics–physiologically based pharmacokinetic (CFD-PBPK) model for ethyl acrylate and its metabolite AA in rats and humans (Frederick et al., 2002) permit use of more information in the dosimetry calculation (U.S. EPA, 1994). First, the interspecies extrapolation of the target tissue dose metrics can be conducted with this model. Secondly, potential pharmacokinetic variability/uncertainty among humans can also be estimated by examining model output for distributions of input parameters.
METHODS
Literature evaluation and preliminary modeling.
Available studies potentially relevant to the determination of a chronic RfC for ethyl acrylate were provided by the sponsor and identified by literature searches conducted by the authors in the areas of ethyl acrylate and AA toxicity and disposition. A critical study, endpoint, mode of action, and an internal dose metric (olfactory concentration of AA) were selected. Preliminary modeling was conducted using the model of Frederick et al. (2002), described in the Appendix. Evaluation of these results in light of the AA literature (described in Results) prompted additional literature evaluation of a potential role for GSH in the mode of action. Because olfactory GSH concentration was identified as a potential basis for chronic RfC derivation, the Frederick et al. (2002) model was refined to improve its ability to describe GSH depletion in the rat olfactory epithelium.
Calculation of AA and GSH Concentrations in Dorsal Olfactory Epithelium
CFD-PBPK model.
Prediction of the AA concentration and GSH concentration in the dorsal olfactory epithelium of a rat or human exposed to a given concentration of ethyl acrylate was performed using a refinement of the CFD-PBPK model developed by Frederick et al. (2002), depicted in Figure 1 and described in the Appendix. Three possible values for the rate of ethyl acrylate metabolism in human nasal tissues were considered (Frederick et al., 2002): the rate determined in human autopsy tissue, the rate determined in monkey tissue, and the rate of ethyl acrylate metabolism rat tissue multiplied by the ratio of rates of methylmethacrylate metabolism in human nasal biopsy samples and in rat tissue (Mainwaring et al., 2001). The adjusted rat tissue rates are the highest (104 and 63 μmol metabolized/ml of tissue/h for olfactory and respiratory epithelium, respectively), and the human tissue rates are the lowest (18.1 and 23.0 μmol metabolized/ml of tissue/h for olfactory and respiratory epithelium). We elected to use the rates determined in human autopsy tissue, because they are derived from human tissue using the compound of concern. In addition, the sample sizes (n) are greater than the sample sizes for other species.
The parameter values used in the current study are the same as those used in previously published CFD-PBPK models for ethyl acrylate and AA (Frederick et al., 1998, 2002) with the following exceptions. For a human engaged in light work, cardiac output is increased from 3.9 to 6.0 ml/sec-kg0.74 (Brown et al., 1997). To facilitate sensitivity analysis, certain flow calculations were modified to ensure mass balance. Specifically, blood flow to the slowly perfused tissues compartment (muscle and fat) was calculated as the difference between the cardiac output and the sum of flows to the liver, kidneys, other richly perfused tissues, nose, and nasopharynx. Likewise, the fraction of the nasal blood flow perfusing the respiratory epithelium was calculated as the difference between total blood flow to the nose and the flow to the olfactory epithelium and the nasal vestibule.
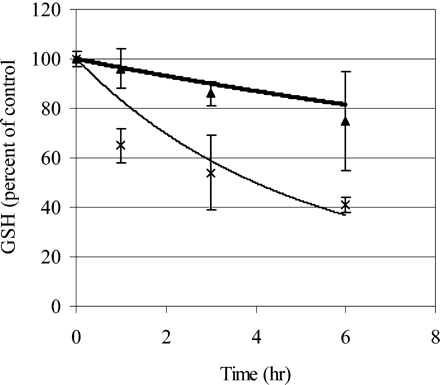
The Frederick et al. (2002) model predicted that the olfactory epithelial concentration of GSH associated with the ethyl acrylate NOEL (5 ppm) after 6 h of exposure would be 95.9% of the initial value. The experimental data, however, indicated greater depletion, to approximately 75% of control at the NOEL and 40% at the LOEL (25 ppm) (Frederick et al., 2002). To use the model for interspecies extrapolation of olfactory GSH concentrations, the rat model was adjusted to provide a more accurate prediction of the measured GSH concentrations. This calibration was accomplished by introducing an adjustment factor for GSH conjugation in the olfactory epithelium (KGSHO) to reflect a rate increase over nonenzymatic rates measured in vitro. A value of KGSHO equal to 10 accurately simulated olfactory GSH concentrations for 6 h exposure of rats to 5 or 25 ppm ethyl acrylate (Fig. 2) without significantly affecting tissue AA predictions. It was assumed that KGSHO would not differ between species. While this adjustment factor, KGSHO, was determined empirically, it is consistent with the literature. Potter and Tran (1993) determined that the rate of second-order conjugation of ethyl acrylate and GSH was increased 15-fold in liver tissue due to the presence of glutathione transferases (GSTs). Banger et al. (1993) determined, using various GST substrates, that the activity of olfactory GSTs ranged from 40 to 90% of that in liver. One would thus anticipate that KGSHO, the increase in GSH conjugation in olfactory epithelium, would be 40 to 90% of 15, or between 6 and 13.5. It is not clear why this increase was not detected in vitro by Frederick et al. (2002).
Computations.
Simulations were performed using ACSL Sim 11.8 (Aegis Technologies Group, Hunstville, AL) on a Dell Optiplex GX260 computer with a Pentium 4 processor or Dell Inspiron 7500 with a Pentium II processor.
Interspecies Extrapolation and Application of Adjustment Factors for CFD-PBPK Modeling Approach
When using an internal dosimetry model, adjustment factors for duration and pharmacodynamic differences are most logically applied to the target internal dose prior to calculation of the human-equivalent continuous exposure concentration (Andersen et al., 2000). The predicted human-equivalent exposure concentration for a human with average pharmacokinetic and physiological parameter values is then determined by iteratively testing different exposure concentrations and calculating internal dose. The resulting human exposure concentration was then used as the basis concentration, and a human pharmacokinetic sensitivity and variability analyses were conducted at this inhaled concentration.
Time adjustment of internal dose.
Area under the curve (AUC), rather than peak concentration, of AA was selected as an appropriate internal dose metric based on the studies of Lomax et al. (1994) that demonstrated that exposures of mice to AA with equivalent concentration × time (C × T) (25 ppm for 4.4 h/day or 5 ppm for 22 h/day) produced similar incidence of nasal irritation. Since steady state concentrations of AA in the olfactory epithelium are expected within 1 h (Frederick et al., 2002), time adjustment for continuous exposure was performed by multiplying the tissue concentration achieved in the rat after 1 h of exposure to ethyl acrylate at the NOEL (5 ppm) and multiplying by (6 h/24 h) × (5 days/7 days) to convert from the schedule in Miller et al. (1985) to account for continuous exposure.
Adjustment factors.
Tissues from individual animals or humans may respond differently to the same concentration of a potentially toxic compound, exhibiting differing pharmacodynamics. The default pharmacodynamic adjustment factor for animal-to-human pharmacodynamic differences is 3 (U.S. EPA, 1994). An adjustment factor for intraspecies (human) variability of 10, to account for pharmacokinetic and pharmacodynamic differences in response to the toxic compound, was also applied. Potentially susceptible subpopulations were considered.
The adjustment factor for intraspecies pharmacokinetic and pharmacodynamic variability was evaluated in light of sensitivity analysis of nasal olfactory epithelium GSH and AA concentrations at the NOEL for rats and at the proposed RfC for humans. Briefly, normalized sensitivity coefficients (SC) were calculated for changes in predicted dose metrics of potential interest for changes in model parameter values. In the analyses presented here, sensitivity (normalized sensitivity coefficient, SC) was determined by increasing the parameter values by 1% and dividing the fractional change in the model prediction by the 1% fractional change in the input parameter.
Variability analysis.
CVi values were calculated from experimental data or estimates taken from the published literature for parameters with |SC| > 0.2. CVm was calculated for the same dose metrics for which the sensitivity analyses were conducted.
RESULTS
Selection of Critical Endpoints and Studies for the Chronic RfC
Miller et al. (1985) identified nasal lesions of the olfactory epithelium in mice and rats that were exposed to 25 but not 5 ppm ethyl acrylate for 6 h/day, 5 days/week for 27 months. Since effects with NOELs higher than the olfactory toxicity NOEL may lead to lower RfCs because of unequal uncertainty factors, all relevant endpoints should be considered. Irritancy at the beginning of exposure and lethargy at the end of exposure were noted in animals exposed to 225 ppm but not 75 ppm ethyl acrylate and the high-dose (225 ppm) animals had significantly decreased (>10%) body weight gains relative to the controls (Miller et al., 1985). Ethyl acrylate does not exhibit selective developmental toxicity (Saillenfait et al., 1999). A two-generation reproduction study of ethyl acrylate has not been conducted, but the lack of reproductive toxicity of AA in rats at doses up to and including 502 mg/kg/day in drinking water (Hellwig et al., 1997) suggests that ethyl acrylate is unlikely to be selectively toxic to reproduction. Hence the relevant endpoints are olfactory lesions, lethargy, irritancy, and body weight.
Selection of Internal Dose Metric for Olfactory Toxicity
Based on the mode of action for nasal damage, measures of internal dose related to AA concentration in the olfactory epithelium were considered. The preferred dose metric was the time-averaged steady state concentration of AA in the nasal olfactory epithelium, rather than the peak concentration. This selection was based on the studies of Lomax et al. (1994) indicating that exposures of mice to AA with equivalent C × T (25 ppm for 4.4 h/day or 5ppm for 22 h/day) produced similar incidence of nasal irritation.
The initial hypothesis was that the AA concentration would be the preferred dose metric for setting a risk reference value for ethyl acrylate. The predicted nasal tissue concentrations of AA at the NOEL were compared for inhalation exposure to AA and for exposure to ethyl acrylate (6 h/day, 5 days/week) using the model of Frederick et al. (2002). The olfactory tissue AA concentrations would be similar if the AA concentration were the sole determinant of nasal damage. In fact, the calculated concentrations differed by a factor of 60 (Table 1).
The comparison of predicted AA concentrations associated with inhalation exposure at the NOELs for AA and ethyl acrylate suggested that AA concentration alone was not sufficient to account for the nasal toxicity of ethyl acrylate. Additional dose measures were considered and tested. These measures included calculated total esterase metabolism and AA concentrations in the blood exchange layer of the nasal epithelial tissues. Upon reviewing the model results (simulations not shown) and the literature on the oral toxicity of ethyl acrylate, it appeared that the most reasonable mode of action for ethyl acrylate toxicity was increased susceptibility to mitochondrial toxicity of AA due to depletion of GSH by conjugation with ethyl acrylate.
A target value for a “safe” olfactory GSH concentration was selected, based on the depletion observed in a single exposure study (Frederick et al., 2002) using the same exposure concentration and duration (5 ppm for 6 h) as the NOEL toxicology study (Miller et al., 1985). A larger body of GSH research (summarized in Frederick et al., 1992), which did not specifically include nasal toxicity, indicated that GSH depletion would have to exceed 50% and be sustained in order to contribute to toxicity. Given the limited experience with role of GSH in nasal toxicity, a clear NOEL for GSH depletion (the experimentally observed value) was used.
Thus, the dose metrics used for interspecies extrapolation were the predicted concentration of AA in the olfactory epithelium (adjusted for exposure duration) and the predicted minimum concentration of GSH in the olfactory epithelium (as percent of control).
Chronic RfC Calculation
In Table 2, approaches to identifying a chronic RfC for ethyl acrylate are summarized. Default approaches to RfCs based on olfactory tissue damage and lethargy/irritancy/body weight decreases (Miller et al., 1985) are based on default U.S. EPA guidance (U.S. EPA, 1994). A chronic RfC based on CFD-PBPK modeling of AA concentrations in olfactory epithelium without consideration of GSH depletion is provided for comparison to the RfC derived when olfactory GSH concentrations were also considered. While uncertainty factors were applied to predicted tissue concentrations of AA, no uncertainty factors were applied to tissue GSH concentrations. GSH depletion per se does not cause the toxicity, but appears to be a modifying pharmacodynamic factor that enhances the toxicity of AA in the tissues.
Sensitivity of Olfactory AA Concentrations to Choice of Work Versus Rest and Human Olfactory Metabolism Rate
The choice of light work versus rest provided a slightly lower value for the RfC. The choice of measured human metabolism rates rather than measured monkey or adjusted rat rates increased the equivalent human ethyl acrylate exposure concentrations (Table 3). These values, however, are still higher than the proposed RfC that incorporated prediction of nasal olfactory GSH depletion, a contributing pharmacodynamic factor.
Sensitivity of Proposed Chronic RfC to Target GSH Concentration
The chronic RfC for olfactory toxicity was derived by determining what exposure concentration would be expected to result in GSH depletion to 75% of control. When 50% GSH depletion rather than 25% depletion was used as the target internal dose metric, the proposed chronic RfC for olfactory toxicity increased from 0.25 ppm to 0.8 ppm. The choice of 25% depletion as a target instead of 50% depletion produced a three-fold (0.8 ppm/0.25 ppm) increase in the RfC for this endpoint.
Sensitivity Analysis of Nasal Olfactory Epithelium AA and GSH Concentrations
The nasal toxicity observed in ethyl acrylate-exposed rats apparently results from enhanced susceptibility to AA toxicity due to GSH depletion. Sensitivity analysis of predicted AA and GSH concentrations in the olfactory epithelium were conducted for the rat at the NOEL (for 6 h of exposure) and the human at the proposed RfC (for 1 week of exposure) to assist in the evaluation of the selected Uncertainty (Adjustment) Factors from Table 2.
The chemical-specific parameters that had the greatest impact on olfactory AA predictions in the rat were the exposure concentration and mucus:air partition coefficient for ethyl acrylate (Table 4). The most sensitive physiological parameters were the width of the olfactory epithelium and mucus thickness over the olfactory epithelium. Model predictions of olfactory tissue GSH concentration after 6 h of exposure were generally less sensitive to model parameters values and were sensitive to exposure concentration, kinetic parameters for ethyl acrylate hydrolysis and GSH conjugation in the olfactory epithelium, the width of the olfactory epithelium, and the mucus:air partition coefficient for ethyl acrylate (Table 5).
Tables 6 and 7 summarize the sensitivity of model predictions of human olfactory AA and GSH concentrations, respectively, to values of model parameters. The chemical-specific parameters that had the greatest impact on tissue AA predictions were the exposure concentration and the nasal tissue:blood partition coefficient for AA. The most sensitive physiological parameters were the respiration rate and tidal volume. Predicted olfactory tissue GSH concentration was generally less sensitive to model parameter values than the AA predictions, but was sensitive to the exposure concentration (proposed RfC), mucus:air partition coefficient for ethyl acrylate, and the parameters describing GSH conjugation and turnover in the olfactory epithelium. The sensitivity analyses indicate that the model predictions upon which the proposed RfC was based, the GSH concentrations, were not sensitive to small changes in most of the model parameter values.
Consideration of Potentially Sensitive Subpopulations
The recommended chronic RfC was determined on the basis of predicted GSH depletion in the olfactory epithelium. As shown in Table 6, olfactory GSH concentrations are most sensitive to olfactory ethyl acrylate-GSH conjugation rate, GSH turnover rate, and the mucus:air partition coefficient for ethyl acrylate. Subpopulations for which these values differ from the population means would not be expected for a thermodynamic parameter, such as the mucus:air partition coefficient, and have not been identified for any of the other parameters. Banger et al. (1996) have demonstrated a lack age- or sex-related differences in cytosolic olfactory GSTs in rats. No additional uncertainty factors are anticipated to be necessary to protect sensitive subpopulations. Based on the variability analysis at the proposed RfC, the predicted concentrations of the putative toxic metabolite AA in human olfactory epithelium for the 95th-percentile individual would be only 2.1-fold higher than in the average individual (1.6 standard deviations higher, with a model CV of 0.71, Table 6), less than the default intraspecies UF for human pharmacokinetic variability (∼3.2). For human olfactory GSH, the variability analysis (Table 7) predicted that the 95th-percentile individual would possess olfactory GSH concentrations of 58% of control. The results of the variability analysis thus indicate that uncertainty factors used for pharmacokinetics (AA concentrations) and pharmacodynamics (GSH concentrations) (Table 2) should provide ample protection of human health.
DISCUSSION
To determine a chronic risk reference value for ethyl acrylate, multiple endpoints and multiple approaches were considered. Because both NOELs and uncertainty/adjustment factors may differ among endpoints, it was necessary to consider both olfactory toxicity and lethargy/irritancy/decreased body weight as possible critical effects. Due to the lack of mechanistic data to support the use of CFD-PBPK modeling for interspecies extrapolation of the lethargy/irritancy/decreased body weight findings, a default approach was used, resulting in an RfC of 0.4 ppm for these endpoints. For the olfactory toxicity, mechanistic data support a predominant role for AA in mitochondrial toxicity leading to inflammation. However, comparisons to results for inhaled AA were inconsistent—ethyl acrylate is more toxic than AA, based on calculations of tissue AA. Based on research showing that ethyl acrylate depletes nasal GSH (Frederick et al., 2002) and that GSH depletion can enhance mitochondrial toxicity of other compounds (Haouzi et al., 2001; Hoek et al., 2002), we developed a theory that ethyl acrylate toxicity results from an enhanced sensitivity to AA due to GSH depletion, and that an appropriate RfC should be based on maintaining sufficient levels of GSH in the olfactory epithelium and preventing elevated tissue levels of AA. Therefore, we developed one olfactory RfC based solely on predicted AA concentrations (RfC = 1.8 ppm) and a second olfactory RfC based on maintaining a protective level of nasal GSH (RfC = 0.25 ppm) and recommend use of the latter value. If only predicted AA concentration had been considered for olfactory toxicity (olfactory RfC = 1.8 ppm), the overall assessment with uncertainty factors totaling 30 would have resulted in a recommended RfC of 0.4 ppm based on lethargy, irritancy, and decreased body weight. Consideration of nasal GSH resulted in a slightly lower RfC of 0.25 ppm.
The recommended chronic RfC of 0.25 ppm for ethyl acrylate is a factor of ∼7 (1.8 ppm/0.25 ppm) lower than the potential RfC derived based on predicted olfactory tissue AA alone (1.8 ppm). The factor of 7 can be considered an additional pharmacodynamic adjustment factor to the predicted AA tissue concentration for the contribution of GSH status to the development of toxicity, in addition to the pharmacodynamic uncertainty factors for AA included in the derivation of the “AA only” RfC (1.8 ppm). The derivation of an RfC of 1.8 ppm based on AA predictions alone (Table 2) included a pharmacodynamic uncertainty factor of 3 for animal to human differences UFA,PD, and an uncertainty factor for differences among humans (UFH = 10) that is generally considered to consist of equal components (∼3.2) for pharmacokinetic and pharmacodynamic variability. The additional pharmacodynamic adjustment factor for GSH (7) accounted for the greater susceptibility, compared to rats, of the human olfactory tissue to GSH depletion predicted by the CFD-PBPK model, and resulted in a total pharmacodynamic uncertainty/adjustment factor = UFA,PD (AA) × UFH,PD (AA) × AFA,PD (GSH) = 3 × 3.2 × 7 = 67.
The sensitivity analysis results for GSH predictions in the rat nose (Table 5) aid in the understanding of why the introduction of KGSHO (enhancement of GSH conjugation in the olfactory tissue by GSTs) improved the predictive capability of the model. The rat olfactory GSH concentrations were sensitive to relatively few model parameters. A reduction in the ethyl acrylate hydrolysis rate was considered, but even if it was set equal to zero, the Frederick et al. (2002) model would predict that GSH would only be depleted to 88% of control (vs. 75% observed in the experiment and 97% predicted in Frederick et al., 2002). Clearly, some or all of the adjustment must be to the GSH modeling.
The model sensitivity analyses indicated that the human nasal olfactory tissue GSH predictions, for which there were no validation data, were not highly sensitive to the model parameter values. A conservative target concentration of GSH was selected, and uncertainty with respect to precisely what level of GSH was necessary to protect human nasal tissue was of less concern due to two factors: the observation that GSH predictions were not highly variable for the population and the prediction that concentrations of the putative toxic metabolite would be seven-fold lower than the target concentrations based on target tissue AA concentration alone.
Model sensitivity analyses of the baseline Frederick et al. (2002) model, without the GSH refinement, were also conducted (data not shown). The sensitivity coefficients for olfactory AA predictions were identical to the refined model, and the results for GSH were very similar, with sensitivity coefficients differing no more that 30% from the values determined for the refined model.
The proposed RfC was derived by considering AA and GSH separately, then comparing the results. That is, a potential RfC was derived based on AA only, and another potential RfC was derived on the basis of GSH only, and this latter value was selected as the proposed RfC because it is also believed to be protective with respect to AA, whereas the “AA-only” RfC may not be sufficiently protective of olfactory GSH for ethyl acrylate-exposed individuals. The possibility of combining the two measures was considered but deemed to be unjustifiable. For example, a lesser extent of GSH depletion and a higher tissue concentration of AA could produce a combined dose metric that is mathematically equivalent to the current proposed RfC, but would logically seem to pose a greater risk to human health because adequate GSH protection would be retained, but concentrations of the putative toxicant would be higher.
On initial review, it appears that the olfactory toxicity RfC based on GSH depletion did not incorporate any uncertainty factors (Table 2). The selection of 25% depletion of olfactory epithelium GSH (equal to the concentration observed at the NOEL in the rat) was somewhat conservative in that other researchers have identified 50% GSH depletion as a threshold for toxicity (Frederick et al., 1992). Also, this olfactory RfC was not based on GSH alone, but on GSH depletion in combination with the presence of AA, which, as noted above, is predicted to be sven-fold lower than a target concentration developed by applying time and uncertainty factor adjustments. As AA was the putative toxicant with GSH as a modifying factor, customary UFs were most logically applied to setting the target values of AA rather than GSH. Some conservatism in the GSH dose metric was warranted, due to the lack of validation data for the human GSH model. The selection of 25% depletion rather than 50% depletion of GSH provided this conservatism and reduced the olfactory toxicity RfC by a factor of approximately 3 (from 0.8 ppm to 0.25 ppm).
The proposed mode of action for olfactory toxicity of ethyl acrylate—AA toxicity enhanced by GSH depletion—is consistent with the mode of action for toxicity of ethyl acrylate for other endpoints and for another olfactory toxicant, methyl iodide. Modes of action for ethyl acrylate tumorigenesis in the rodent forestomach and acute lethality have both been linked to GSH depletion (Frederick et al., 1992, 2002). Chamberlain et al. (1998) have investigated the mode of action for olfactory toxicity of methyl iodide in rats by modulating the status of GSH and cytochrome P450. They concluded that GSH depletion (to 40% of control), rather than GSH-metabolite formation, initiated nasal toxicity, and that the relatively slow turnover of GSH in the olfactory epithelium (compared to respiratory epithelium and other tissues) prolonged GSH depletion and rendered olfactory epithelium more susceptible to oxidative damage than the respiratory epithelium. Ethyl acrylate was hydrolyzed to AA at similar rates in nasal olfactory and respiratory epithelium, and the tissues have similar GSH concentrations following single exposures, but toxicity was limited to the olfactory region (Frederick et al., 2002; Miller et al., 1985), indicating that the slow recovery of GSH concentrations after exposure may contribute to the development of the nasal olfactory lesions.
Comparisons of ethyl acrylate nasal toxicities between species, including consideration of acrylic acid, can be informative as to possible mechanisms of action. Our initial hypothesis was that the nasal lesions produced following ethyl acrylate exposure were due to the formation of AA. Comparisons of the concentrations of AA predicted by modeling (see Table 1) indicated that other factors in addition to AA concentration were involved in the toxicity (e.g., GSH depletion). Inspection of the NOELs and LOELs for EA and AA in rats and mice also indicated factors other than AA production might be involved in the formation of nasal lesions following ethyl acrylate exposures (Miller et al., 1985). The NOELs and LOELs for mice and rats exposed chronically to ethyl acrylate are both 5 ppm and 25 ppm, respectively. The NOEL and LOEL for chronic AA exposures in rats are 25 ppm and 75 ppm, values that are higher than those for ethyl acrylate indicating that the ester may be more potent than the acid alone (Miller et al., 1981). The LOELs for mice exposed to AA were 5 ppm for female mice and 25 ppm for male mice, providing no clear indication that the ester might be more potent.
In summary, a proposed RfC for ethyl acrylate was developed based not only on the expected concentrations of the toxic metabolite AA, but also on GSH, which serves both as a cofactor for ethyl acrylate metabolism and as an indicator of tissue oxidative stress. The proposed RfC of 0.25 ppm is lower than the RfC that would have been calculated based on AA prediction alone (1.8 ppm), but 50-fold higher than the RfC yielded by the default approach, 0.005 ppm.
APPENDIX
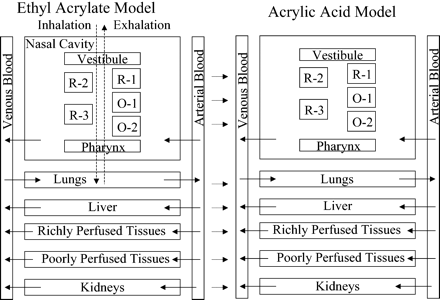
The ethyl acrylate model (Fig. 1) was an extension of the CFD-PBPK model for AA (Andersen et al., 2000; Bush et al., 1998; Frederick et al., 1998). The Frederick et al. (2002) model incorporated cyclic breathing (i.e., inhalation followed by exhalation), airflow through the nasal passages, mass transfer of ethyl acrylate from the vapor phase to the mucus layer, diffusion from the mucus layer to the tissue layers, and diffusion through tissue layers (four for olfactory epithelium, two for respiratory epithelium, one for remaining nasal surfaces) to the blood exchange region. The model described the transport of ethyl acrylate through the blood stream to the lungs, liver, kidneys, other richly perfused tissues, and poorly perfused tissues (muscle and fat), which were modeled as single, well-mixed compartments. The same model structure was used to describe the distribution of metabolically generated AA, with the exception that exhalation of the acid was expected to be negligible (rat blood:air partition coefficient = 4300 ± 130; Frederick et al., 1998) and was neglected by Frederick et al. (2002). Metabolism of ethyl acrylate occurred through two pathways—second-order conjugation with GSH or saturable (Michaelis-Menten) esterase-mediated hydrolysis to AA. AA metabolism occurred through saturable oxidation.
Scaling of Metabolic Parameters
As previously described (Frederick et al., 1998, 2002), rates of ethyl acrylate and AA metabolism in lung, liver, kidney, other richly perfused tissues, and muscle were scaled from in vitro (per ml of tissue, Black and Finch, 1995; Frederick et al., 2002) to in vivo, based on tissue volume. Metabolic rates for nasal olfactory and respiratory epithelium were scaled, based on tissue volume, and adjusted for in vitro dilution of metabolically active tissues with connective tissues, based on microscopic examination of nasal epithelial samples (Frederick et al., 2002). In vitro rates of AA metabolism determined in rat tissues (Black and Finch, 1995) were used for both rats and humans. In vitro rates of ethyl acrylate hydrolysis were determined in rat, monkey, and human tissues (Frederick et al., 2002).
Model equations have previously been provided by Frederick et al. (1998). Model code (in ACSL) will be provided by the corresponding author upon request.
RGDOEij = Rate of GSH conjugation in dorsal olfactory epithelium tissue compartment j, layer i (μmol/h).
VDOEij = Volume of dorsal olfactory epithelium tissue compartment j, layer i (ml).
KGSH = Second-order rate of nonenzymatic GSH conjugation (ml/h-μmol).
Kgsho = increase in GSH conjugation rate relative to nonenzymatic rate, olfactory tissue (dimensionless).
GSDOEij = GSH concentration in dorsal olfactory epithelium tissue compartment j, layer i (μmol/ml)
CDOEij = Ethyl acrylate concentration in dorsal olfactory epithelium tissue compartment j, layer i (μmol /ml).
Computational fluid dynamics-physiologically based pharmacokinetic model for ethyl acrylate and its metabolite acrylic acid (AA) in rats and humans. R = respiratory epithelium, O = olfactory epithelium. Solid arrows denote blood flow, arrows with large dashes denote air flow (cyclic breathing), and dotted arrows denote metabolism. Adapted from Frederick et al. (2002).
Glutathione (GSH) concentration (percent of control) in the olfactory epithelium of the dorsal meatus of rats exposed to ethyl acrylate by inhalation. Lines: Model predictions for 5 ppm (top, thick line), 25 ppm (bottom, thin line), refinement of Frederick et al. (2002) model. Experimental data of Frederick et al. (2002) (mean ± SD)> = 5 ppm, × = 25 ppm.
Acrylic Acid (AA) Concentrations Associated with No Observed Effect Levels and Lowest Observed Effect Levels for Olfactory Tissue Damage in Rats or Rat Tissue
| Endpoint for rats/rat tissues . | Associated AA concentration . |
|---|---|
| AA NOEL, LOEL in vivo | 3 mM, 6 mM (Frederick et al., 1998; Andersen et al., 2000) |
| Ethyl acrylate NOEL, LOEL in vivo | 0.05 mM, 0.25 mM |
| AA NOEL, LOEL in vitro* | 0.4 mM, 0.6 mM (Frederick et al., 1998) |
| Endpoint for rats/rat tissues . | Associated AA concentration . |
|---|---|
| AA NOEL, LOEL in vivo | 3 mM, 6 mM (Frederick et al., 1998; Andersen et al., 2000) |
| Ethyl acrylate NOEL, LOEL in vivo | 0.05 mM, 0.25 mM |
| AA NOEL, LOEL in vitro* | 0.4 mM, 0.6 mM (Frederick et al., 1998) |
Rat nasal explants
Acrylic Acid (AA) Concentrations Associated with No Observed Effect Levels and Lowest Observed Effect Levels for Olfactory Tissue Damage in Rats or Rat Tissue
| Endpoint for rats/rat tissues . | Associated AA concentration . |
|---|---|
| AA NOEL, LOEL in vivo | 3 mM, 6 mM (Frederick et al., 1998; Andersen et al., 2000) |
| Ethyl acrylate NOEL, LOEL in vivo | 0.05 mM, 0.25 mM |
| AA NOEL, LOEL in vitro* | 0.4 mM, 0.6 mM (Frederick et al., 1998) |
| Endpoint for rats/rat tissues . | Associated AA concentration . |
|---|---|
| AA NOEL, LOEL in vivo | 3 mM, 6 mM (Frederick et al., 1998; Andersen et al., 2000) |
| Ethyl acrylate NOEL, LOEL in vivo | 0.05 mM, 0.25 mM |
| AA NOEL, LOEL in vitro* | 0.4 mM, 0.6 mM (Frederick et al., 1998) |
Rat nasal explants
Default and Computational Fluid Dynamics-Physiologically Based Pharmacokinetic Modeling Approaches to Selection of a Chronic Reference Concentration for Inhaled Ethyl Acrylate
| . | Endpoint (extrapolation approach) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Lethargy, irritancy, decreased body weight . | Olfactory tissue damage . | . | . | . | ||||
| . | Default . | Default . | CFD-PBPK, AA . | CFD-PBPK, GSH, and AA . | CFD-PBPK, GSH and AA . | ||||
| NOEL (ppm) (Miller et al., 1985) | 75 | 5 | 5 | 5 | 5 | ||||
| LOEL (ppm) (Miller et al., 1985) | 225 | 25 | 25 | 25 | 25 | ||||
| Dose Measure | external concentration | external concentration | 0.05 mM AA in olfactory epitheliuma | minimum olfactory tissuea GSH = 50% of control | minimum olfactory tissuea GSH = 50% of control | ||||
| Duration adjustment (h per day, days per week) | (6/24) × (5/7) | (6/24) × (5/7) | (6/24) × (5/7) | NA | NA | ||||
| Regional gas dosimetry ratio (RGDR) | 1 (Category 2, systemic effect at higher concentrations) | 0.18 (Category 1, portal of entry effect only at low concentration) | NA | NA | NA | ||||
| UFA,PD | 3 | 3 | 3 | NA | NA | ||||
| UFH | 10 | 10 | 10 | NA | NA | ||||
| Target internal concentration for human CFD-PBPK Modela | NA | NA | 0.05 mM AA × (6/24) × (5/7)/(3 × 10) = 0.0003 mM AA in olfactory epitheliuma | minimum olfactory tissuea GSH = 50% ofcontrol | minimum olfactory tissuea GSH = 75% of control | ||||
| RfC calculationb | 75 ppm × (6/24) × (5/7) × 1/(3 × 10) | 5 ppm × (6/24) × (5/7) × 0.18/(3 × 10) | human CFD-PBPK modelc | human CFD-PBPK modelc | human CFD-PBPK modelc | ||||
| Chronic RfC | 0.4 ppm | 0.005 ppm | 1.8 ppm | 0.8 ppm | 0.25 ppm (Recommended RfC) | ||||
| . | Endpoint (extrapolation approach) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Lethargy, irritancy, decreased body weight . | Olfactory tissue damage . | . | . | . | ||||
| . | Default . | Default . | CFD-PBPK, AA . | CFD-PBPK, GSH, and AA . | CFD-PBPK, GSH and AA . | ||||
| NOEL (ppm) (Miller et al., 1985) | 75 | 5 | 5 | 5 | 5 | ||||
| LOEL (ppm) (Miller et al., 1985) | 225 | 25 | 25 | 25 | 25 | ||||
| Dose Measure | external concentration | external concentration | 0.05 mM AA in olfactory epitheliuma | minimum olfactory tissuea GSH = 50% of control | minimum olfactory tissuea GSH = 50% of control | ||||
| Duration adjustment (h per day, days per week) | (6/24) × (5/7) | (6/24) × (5/7) | (6/24) × (5/7) | NA | NA | ||||
| Regional gas dosimetry ratio (RGDR) | 1 (Category 2, systemic effect at higher concentrations) | 0.18 (Category 1, portal of entry effect only at low concentration) | NA | NA | NA | ||||
| UFA,PD | 3 | 3 | 3 | NA | NA | ||||
| UFH | 10 | 10 | 10 | NA | NA | ||||
| Target internal concentration for human CFD-PBPK Modela | NA | NA | 0.05 mM AA × (6/24) × (5/7)/(3 × 10) = 0.0003 mM AA in olfactory epitheliuma | minimum olfactory tissuea GSH = 50% ofcontrol | minimum olfactory tissuea GSH = 75% of control | ||||
| RfC calculationb | 75 ppm × (6/24) × (5/7) × 1/(3 × 10) | 5 ppm × (6/24) × (5/7) × 0.18/(3 × 10) | human CFD-PBPK modelc | human CFD-PBPK modelc | human CFD-PBPK modelc | ||||
| Chronic RfC | 0.4 ppm | 0.005 ppm | 1.8 ppm | 0.8 ppm | 0.25 ppm (Recommended RfC) | ||||
Dorsal olfactory epithelium, first tissue layer below mucus.
Default RfC = NOEL × Time Adjustment × RGDR/(UFA,PD × UFH).
CFD-PBPK model of Frederick et al. (2002), modified as described under “Methods,” assuming light work.
Default and Computational Fluid Dynamics-Physiologically Based Pharmacokinetic Modeling Approaches to Selection of a Chronic Reference Concentration for Inhaled Ethyl Acrylate
| . | Endpoint (extrapolation approach) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Lethargy, irritancy, decreased body weight . | Olfactory tissue damage . | . | . | . | ||||
| . | Default . | Default . | CFD-PBPK, AA . | CFD-PBPK, GSH, and AA . | CFD-PBPK, GSH and AA . | ||||
| NOEL (ppm) (Miller et al., 1985) | 75 | 5 | 5 | 5 | 5 | ||||
| LOEL (ppm) (Miller et al., 1985) | 225 | 25 | 25 | 25 | 25 | ||||
| Dose Measure | external concentration | external concentration | 0.05 mM AA in olfactory epitheliuma | minimum olfactory tissuea GSH = 50% of control | minimum olfactory tissuea GSH = 50% of control | ||||
| Duration adjustment (h per day, days per week) | (6/24) × (5/7) | (6/24) × (5/7) | (6/24) × (5/7) | NA | NA | ||||
| Regional gas dosimetry ratio (RGDR) | 1 (Category 2, systemic effect at higher concentrations) | 0.18 (Category 1, portal of entry effect only at low concentration) | NA | NA | NA | ||||
| UFA,PD | 3 | 3 | 3 | NA | NA | ||||
| UFH | 10 | 10 | 10 | NA | NA | ||||
| Target internal concentration for human CFD-PBPK Modela | NA | NA | 0.05 mM AA × (6/24) × (5/7)/(3 × 10) = 0.0003 mM AA in olfactory epitheliuma | minimum olfactory tissuea GSH = 50% ofcontrol | minimum olfactory tissuea GSH = 75% of control | ||||
| RfC calculationb | 75 ppm × (6/24) × (5/7) × 1/(3 × 10) | 5 ppm × (6/24) × (5/7) × 0.18/(3 × 10) | human CFD-PBPK modelc | human CFD-PBPK modelc | human CFD-PBPK modelc | ||||
| Chronic RfC | 0.4 ppm | 0.005 ppm | 1.8 ppm | 0.8 ppm | 0.25 ppm (Recommended RfC) | ||||
| . | Endpoint (extrapolation approach) . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| . | Lethargy, irritancy, decreased body weight . | Olfactory tissue damage . | . | . | . | ||||
| . | Default . | Default . | CFD-PBPK, AA . | CFD-PBPK, GSH, and AA . | CFD-PBPK, GSH and AA . | ||||
| NOEL (ppm) (Miller et al., 1985) | 75 | 5 | 5 | 5 | 5 | ||||
| LOEL (ppm) (Miller et al., 1985) | 225 | 25 | 25 | 25 | 25 | ||||
| Dose Measure | external concentration | external concentration | 0.05 mM AA in olfactory epitheliuma | minimum olfactory tissuea GSH = 50% of control | minimum olfactory tissuea GSH = 50% of control | ||||
| Duration adjustment (h per day, days per week) | (6/24) × (5/7) | (6/24) × (5/7) | (6/24) × (5/7) | NA | NA | ||||
| Regional gas dosimetry ratio (RGDR) | 1 (Category 2, systemic effect at higher concentrations) | 0.18 (Category 1, portal of entry effect only at low concentration) | NA | NA | NA | ||||
| UFA,PD | 3 | 3 | 3 | NA | NA | ||||
| UFH | 10 | 10 | 10 | NA | NA | ||||
| Target internal concentration for human CFD-PBPK Modela | NA | NA | 0.05 mM AA × (6/24) × (5/7)/(3 × 10) = 0.0003 mM AA in olfactory epitheliuma | minimum olfactory tissuea GSH = 50% ofcontrol | minimum olfactory tissuea GSH = 75% of control | ||||
| RfC calculationb | 75 ppm × (6/24) × (5/7) × 1/(3 × 10) | 5 ppm × (6/24) × (5/7) × 0.18/(3 × 10) | human CFD-PBPK modelc | human CFD-PBPK modelc | human CFD-PBPK modelc | ||||
| Chronic RfC | 0.4 ppm | 0.005 ppm | 1.8 ppm | 0.8 ppm | 0.25 ppm (Recommended RfC) | ||||
Dorsal olfactory epithelium, first tissue layer below mucus.
Default RfC = NOEL × Time Adjustment × RGDR/(UFA,PD × UFH).
CFD-PBPK model of Frederick et al. (2002), modified as described under “Methods,” assuming light work.
Ethyl Acrylate Exposure Concentrations Corresponding to Target Internal Concentrations of Acrylic Acid in the Nasal Epithelium
| . | At Rest . | Light worka . |
|---|---|---|
| Measured human nasal metabolism ratesb | 2.2 ppm | 1.8 ppm |
| Measured monkey nasal metabolism ratesb | 0.9 ppm | 0.8 ppm |
| Adjusted rat nasal metabolism ratesc | 0.6 ppm | 0.6 ppm |
| . | At Rest . | Light worka . |
|---|---|---|
| Measured human nasal metabolism ratesb | 2.2 ppm | 1.8 ppm |
| Measured monkey nasal metabolism ratesb | 0.9 ppm | 0.8 ppm |
| Adjusted rat nasal metabolism ratesc | 0.6 ppm | 0.6 ppm |
Approximately 50 W.
Ethyl acrylate metabolism in nasal respiratory and olfactory epithelium in vitro (Frederick et al., 2002).
Ethyl acrylate metabolism rates measured in rat tissue (Frederick et al., 2002), adjusted by human:rat metabolism ratio for methyl methacrylate (Mainwaring et al., 2001).
Ethyl Acrylate Exposure Concentrations Corresponding to Target Internal Concentrations of Acrylic Acid in the Nasal Epithelium
| . | At Rest . | Light worka . |
|---|---|---|
| Measured human nasal metabolism ratesb | 2.2 ppm | 1.8 ppm |
| Measured monkey nasal metabolism ratesb | 0.9 ppm | 0.8 ppm |
| Adjusted rat nasal metabolism ratesc | 0.6 ppm | 0.6 ppm |
| . | At Rest . | Light worka . |
|---|---|---|
| Measured human nasal metabolism ratesb | 2.2 ppm | 1.8 ppm |
| Measured monkey nasal metabolism ratesb | 0.9 ppm | 0.8 ppm |
| Adjusted rat nasal metabolism ratesc | 0.6 ppm | 0.6 ppm |
Approximately 50 W.
Ethyl acrylate metabolism in nasal respiratory and olfactory epithelium in vitro (Frederick et al., 2002).
Ethyl acrylate metabolism rates measured in rat tissue (Frederick et al., 2002), adjusted by human:rat metabolism ratio for methyl methacrylate (Mainwaring et al., 2001).
Sensitivity of Predictions of Acrylic Acid (AA) Concentration in the First Layer of the Rat Dorsal Nasal Olfactory Epithelium to Model Parameter Values
| Parametera . | Normalized sensitivity coefficient . |
|---|---|
| Exposure concentration | 1.00 |
| Mucus:air partition coefficient for ethyl acrylate | 0.89 |
| Diffusivity in olfactory epithelium | −0.42 |
\({1}/{4}\) of distance across the olfactory epithelium | 0.41 |
| Thickness of mucus over olfactory epithelium | −0.38 |
| Mucus diffusivity | 0.35 |
| Mucus:epithelium partition coefficient for ethyl acrylate | −0.32 |
| Nasal tissue:blood partition coefficient for ethyl acrylate | 0.27 |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.27 |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.27 |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.27 |
| Width of blood exchange region under olfactory epithelium | 0.26 |
| Nasal tissue:blood partition coefficient for AA | 0.24 |
| Normalized cardiac output | −0.23 |
| Fraction of nasal blood flow to olfactory epithelium in anterior region | −0.21 |
| Fraction of nasal blood flow to olfactory epithelium | −0.21 |
| Blood flow to nasal cavity (fraction of cardiac output) | −0.21 |
| Body weight | −0.20 |
| Parametera . | Normalized sensitivity coefficient . |
|---|---|
| Exposure concentration | 1.00 |
| Mucus:air partition coefficient for ethyl acrylate | 0.89 |
| Diffusivity in olfactory epithelium | −0.42 |
\({1}/{4}\) of distance across the olfactory epithelium | 0.41 |
| Thickness of mucus over olfactory epithelium | −0.38 |
| Mucus diffusivity | 0.35 |
| Mucus:epithelium partition coefficient for ethyl acrylate | −0.32 |
| Nasal tissue:blood partition coefficient for ethyl acrylate | 0.27 |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.27 |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.27 |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.27 |
| Width of blood exchange region under olfactory epithelium | 0.26 |
| Nasal tissue:blood partition coefficient for AA | 0.24 |
| Normalized cardiac output | −0.23 |
| Fraction of nasal blood flow to olfactory epithelium in anterior region | −0.21 |
| Fraction of nasal blood flow to olfactory epithelium | −0.21 |
| Blood flow to nasal cavity (fraction of cardiac output) | −0.21 |
| Body weight | −0.20 |
Simulations conducted with the refined CFD-PBPK model for cyclic breathing of 5 ppm ethyl acrylate by rats for 1 h (steady-state AA). Only parameters with |normalized sensitivity coefficient| > 0.20 are shown. An additional 10 parameters had |normalized sensitivity coefficient| between 0.05 and 0.20, with the remaining 100 parameters having |normalized sensitivity coefficient| < 0.05.
Sensitivity of Predictions of Acrylic Acid (AA) Concentration in the First Layer of the Rat Dorsal Nasal Olfactory Epithelium to Model Parameter Values
| Parametera . | Normalized sensitivity coefficient . |
|---|---|
| Exposure concentration | 1.00 |
| Mucus:air partition coefficient for ethyl acrylate | 0.89 |
| Diffusivity in olfactory epithelium | −0.42 |
\({1}/{4}\) of distance across the olfactory epithelium | 0.41 |
| Thickness of mucus over olfactory epithelium | −0.38 |
| Mucus diffusivity | 0.35 |
| Mucus:epithelium partition coefficient for ethyl acrylate | −0.32 |
| Nasal tissue:blood partition coefficient for ethyl acrylate | 0.27 |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.27 |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.27 |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.27 |
| Width of blood exchange region under olfactory epithelium | 0.26 |
| Nasal tissue:blood partition coefficient for AA | 0.24 |
| Normalized cardiac output | −0.23 |
| Fraction of nasal blood flow to olfactory epithelium in anterior region | −0.21 |
| Fraction of nasal blood flow to olfactory epithelium | −0.21 |
| Blood flow to nasal cavity (fraction of cardiac output) | −0.21 |
| Body weight | −0.20 |
| Parametera . | Normalized sensitivity coefficient . |
|---|---|
| Exposure concentration | 1.00 |
| Mucus:air partition coefficient for ethyl acrylate | 0.89 |
| Diffusivity in olfactory epithelium | −0.42 |
\({1}/{4}\) of distance across the olfactory epithelium | 0.41 |
| Thickness of mucus over olfactory epithelium | −0.38 |
| Mucus diffusivity | 0.35 |
| Mucus:epithelium partition coefficient for ethyl acrylate | −0.32 |
| Nasal tissue:blood partition coefficient for ethyl acrylate | 0.27 |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.27 |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.27 |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.27 |
| Width of blood exchange region under olfactory epithelium | 0.26 |
| Nasal tissue:blood partition coefficient for AA | 0.24 |
| Normalized cardiac output | −0.23 |
| Fraction of nasal blood flow to olfactory epithelium in anterior region | −0.21 |
| Fraction of nasal blood flow to olfactory epithelium | −0.21 |
| Blood flow to nasal cavity (fraction of cardiac output) | −0.21 |
| Body weight | −0.20 |
Simulations conducted with the refined CFD-PBPK model for cyclic breathing of 5 ppm ethyl acrylate by rats for 1 h (steady-state AA). Only parameters with |normalized sensitivity coefficient| > 0.20 are shown. An additional 10 parameters had |normalized sensitivity coefficient| between 0.05 and 0.20, with the remaining 100 parameters having |normalized sensitivity coefficient| < 0.05.
Sensitivity of Predictions of Glutathione (GSH) Concentration in the First Layer of the Rat Dorsal Nasal Olfactory Epithelium to Model Parameter Values
| Parametera . | Normalized sensitivity coefficient . |
|---|---|
\({1}/{4}\) of distance across the olfactory epithelium | 0.30 |
| Exposure concentration | −0.29 |
| Augmentation of GSH conjugation in olfactory epithelium by GSH transferases | −0.28 |
| Nonenzymatic second order rate constant for GSH conjugation with ethyl acrylate | −0.28 |
| Mucus:air partition coefficient for ethyl acrylate | −0.26 |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.21 |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.21 |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.20 |
| Parametera . | Normalized sensitivity coefficient . |
|---|---|
\({1}/{4}\) of distance across the olfactory epithelium | 0.30 |
| Exposure concentration | −0.29 |
| Augmentation of GSH conjugation in olfactory epithelium by GSH transferases | −0.28 |
| Nonenzymatic second order rate constant for GSH conjugation with ethyl acrylate | −0.28 |
| Mucus:air partition coefficient for ethyl acrylate | −0.26 |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.21 |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.21 |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.20 |
Simulations conducted with the refined CFD-PBPK model for cyclic breathing of 5 ppm ethyl acrylate by rats for 6 h. Only parameters with |normalized sensitivity coefficient| > 0.20 are shown. An additional 4 parameters had |normalized sensitivity coefficient| between 0.05 and 0.20, with the remaining 113 parameters having |normalized sensitivity coefficient| < 0.05.
Sensitivity of Predictions of Glutathione (GSH) Concentration in the First Layer of the Rat Dorsal Nasal Olfactory Epithelium to Model Parameter Values
| Parametera . | Normalized sensitivity coefficient . |
|---|---|
\({1}/{4}\) of distance across the olfactory epithelium | 0.30 |
| Exposure concentration | −0.29 |
| Augmentation of GSH conjugation in olfactory epithelium by GSH transferases | −0.28 |
| Nonenzymatic second order rate constant for GSH conjugation with ethyl acrylate | −0.28 |
| Mucus:air partition coefficient for ethyl acrylate | −0.26 |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.21 |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.21 |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.20 |
| Parametera . | Normalized sensitivity coefficient . |
|---|---|
\({1}/{4}\) of distance across the olfactory epithelium | 0.30 |
| Exposure concentration | −0.29 |
| Augmentation of GSH conjugation in olfactory epithelium by GSH transferases | −0.28 |
| Nonenzymatic second order rate constant for GSH conjugation with ethyl acrylate | −0.28 |
| Mucus:air partition coefficient for ethyl acrylate | −0.26 |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.21 |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.21 |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.20 |
Simulations conducted with the refined CFD-PBPK model for cyclic breathing of 5 ppm ethyl acrylate by rats for 6 h. Only parameters with |normalized sensitivity coefficient| > 0.20 are shown. An additional 4 parameters had |normalized sensitivity coefficient| between 0.05 and 0.20, with the remaining 113 parameters having |normalized sensitivity coefficient| < 0.05.
Sensitivity of Predictions of AA Concentration in the First Layer of the Human Dorsal Nasal Olfactory Epithelium to Model Parameter Values and Predicted Variability
| Parametera . | Normalized sensitivity coefficient . | Parameter coefficient of variation . |
|---|---|---|
| Exposure concentration | 1.00 | not applicable |
| Respiration rate | 0.57 | 0.1b |
| Tidal volume | 0.57 | 0.33b |
| Body weight | −0.55 | 0.15c |
| Nasal tissue:blood partition coefficient for AA | 0.54 | 0.19d |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.52 | 0.95e |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.52 | 0.3f |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.51 | 0.61e |
| Width of olfactory epithelium | 0.46 | 0.2g |
| Mucus:air partition coefficient for ethyl acrylate | 0.45 | 0.24e |
| Diffusivity in olfactory epithelium | −0.45 | 0.56h |
\({1}/{4}\) of distance across the olfactory epithelium | 0.27 | 0.2g |
| Nasal tissue:blood partition coefficient for ethyl acrylate | 0.23 | 0.17e |
| Model Coefficient of Variation | 0.71 |
| Parametera . | Normalized sensitivity coefficient . | Parameter coefficient of variation . |
|---|---|---|
| Exposure concentration | 1.00 | not applicable |
| Respiration rate | 0.57 | 0.1b |
| Tidal volume | 0.57 | 0.33b |
| Body weight | −0.55 | 0.15c |
| Nasal tissue:blood partition coefficient for AA | 0.54 | 0.19d |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.52 | 0.95e |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.52 | 0.3f |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.51 | 0.61e |
| Width of olfactory epithelium | 0.46 | 0.2g |
| Mucus:air partition coefficient for ethyl acrylate | 0.45 | 0.24e |
| Diffusivity in olfactory epithelium | −0.45 | 0.56h |
\({1}/{4}\) of distance across the olfactory epithelium | 0.27 | 0.2g |
| Nasal tissue:blood partition coefficient for ethyl acrylate | 0.23 | 0.17e |
| Model Coefficient of Variation | 0.71 |
Simulations conducted with the refined CFD-PBPK model for cyclic breathing of 0.25 ppm ethyl acrylate by humans for 1 week (steady-state). Only parameters with |normalized sensitivity coefficient| > 0.20 are shown. An additional 31 parameters had |normalized sensitivity coefficient| between 0.05 and 0.20, with the remaining 85 parameters having |normalized sensitivity coefficient| < 0.05.
Sensitivity of Predictions of AA Concentration in the First Layer of the Human Dorsal Nasal Olfactory Epithelium to Model Parameter Values and Predicted Variability
| Parametera . | Normalized sensitivity coefficient . | Parameter coefficient of variation . |
|---|---|---|
| Exposure concentration | 1.00 | not applicable |
| Respiration rate | 0.57 | 0.1b |
| Tidal volume | 0.57 | 0.33b |
| Body weight | −0.55 | 0.15c |
| Nasal tissue:blood partition coefficient for AA | 0.54 | 0.19d |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.52 | 0.95e |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.52 | 0.3f |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.51 | 0.61e |
| Width of olfactory epithelium | 0.46 | 0.2g |
| Mucus:air partition coefficient for ethyl acrylate | 0.45 | 0.24e |
| Diffusivity in olfactory epithelium | −0.45 | 0.56h |
\({1}/{4}\) of distance across the olfactory epithelium | 0.27 | 0.2g |
| Nasal tissue:blood partition coefficient for ethyl acrylate | 0.23 | 0.17e |
| Model Coefficient of Variation | 0.71 |
| Parametera . | Normalized sensitivity coefficient . | Parameter coefficient of variation . |
|---|---|---|
| Exposure concentration | 1.00 | not applicable |
| Respiration rate | 0.57 | 0.1b |
| Tidal volume | 0.57 | 0.33b |
| Body weight | −0.55 | 0.15c |
| Nasal tissue:blood partition coefficient for AA | 0.54 | 0.19d |
| Ethyl acrylate hydrolysis capacity in nasal olfactory epithelium | 0.52 | 0.95e |
| Dilution of enzymatically rich layers of nasal olfactory epithelium in vitro | 0.52 | 0.3f |
| Enzyme affinity for ethyl acrylate hydrolysis in the nasal olfactory epithelium | −0.51 | 0.61e |
| Width of olfactory epithelium | 0.46 | 0.2g |
| Mucus:air partition coefficient for ethyl acrylate | 0.45 | 0.24e |
| Diffusivity in olfactory epithelium | −0.45 | 0.56h |
\({1}/{4}\) of distance across the olfactory epithelium | 0.27 | 0.2g |
| Nasal tissue:blood partition coefficient for ethyl acrylate | 0.23 | 0.17e |
| Model Coefficient of Variation | 0.71 |
Simulations conducted with the refined CFD-PBPK model for cyclic breathing of 0.25 ppm ethyl acrylate by humans for 1 week (steady-state). Only parameters with |normalized sensitivity coefficient| > 0.20 are shown. An additional 31 parameters had |normalized sensitivity coefficient| between 0.05 and 0.20, with the remaining 85 parameters having |normalized sensitivity coefficient| < 0.05.
Sensitivity of Predictions of GSH Concentration (Percent of Control) in the First Layer of the Human Dorsal Nasal Olfactory Epithelium to Model Parameter Values and Predicted Variability
| Parametera . | Normalized sensitivity coefficient . | Parameter coefficient of variation . |
|---|---|---|
| Exposure concentration | −0.26 | not applicable |
| Augmentation of GSH conjugation in olfactory epithelium by GSH transferases | −0.26 | 0.3b |
| Nonenzymatic second order rate constant for GSH conjugation with ethyl acrylate | −0.26 | 0.3c |
| Mucus:air partition coefficient for ethyl acrylate | −0.24 | 0.24c |
| First-order GSH loss in the nasal olfactory epithelium | 0.24 | 0.3c |
| Model Coefficient of Variation | 0.14 |
| Parametera . | Normalized sensitivity coefficient . | Parameter coefficient of variation . |
|---|---|---|
| Exposure concentration | −0.26 | not applicable |
| Augmentation of GSH conjugation in olfactory epithelium by GSH transferases | −0.26 | 0.3b |
| Nonenzymatic second order rate constant for GSH conjugation with ethyl acrylate | −0.26 | 0.3c |
| Mucus:air partition coefficient for ethyl acrylate | −0.24 | 0.24c |
| First-order GSH loss in the nasal olfactory epithelium | 0.24 | 0.3c |
| Model Coefficient of Variation | 0.14 |
Simulations conducted with the refined CFD-PBPK model for cyclic breathing of 0.25 ppm ethyl acrylate by humans for 1 week (steady-state). Only parameters with |normalized sensitivity coefficient| > 0.20 are shown. The remaining 124 parameters had |normalized sensitivity coefficient| < 0.05.
Sensitivity of Predictions of GSH Concentration (Percent of Control) in the First Layer of the Human Dorsal Nasal Olfactory Epithelium to Model Parameter Values and Predicted Variability
| Parametera . | Normalized sensitivity coefficient . | Parameter coefficient of variation . |
|---|---|---|
| Exposure concentration | −0.26 | not applicable |
| Augmentation of GSH conjugation in olfactory epithelium by GSH transferases | −0.26 | 0.3b |
| Nonenzymatic second order rate constant for GSH conjugation with ethyl acrylate | −0.26 | 0.3c |
| Mucus:air partition coefficient for ethyl acrylate | −0.24 | 0.24c |
| First-order GSH loss in the nasal olfactory epithelium | 0.24 | 0.3c |
| Model Coefficient of Variation | 0.14 |
| Parametera . | Normalized sensitivity coefficient . | Parameter coefficient of variation . |
|---|---|---|
| Exposure concentration | −0.26 | not applicable |
| Augmentation of GSH conjugation in olfactory epithelium by GSH transferases | −0.26 | 0.3b |
| Nonenzymatic second order rate constant for GSH conjugation with ethyl acrylate | −0.26 | 0.3c |
| Mucus:air partition coefficient for ethyl acrylate | −0.24 | 0.24c |
| First-order GSH loss in the nasal olfactory epithelium | 0.24 | 0.3c |
| Model Coefficient of Variation | 0.14 |
Simulations conducted with the refined CFD-PBPK model for cyclic breathing of 0.25 ppm ethyl acrylate by humans for 1 week (steady-state). Only parameters with |normalized sensitivity coefficient| > 0.20 are shown. The remaining 124 parameters had |normalized sensitivity coefficient| < 0.05.
This work was funded by the Basic Acrylic Monomers Manufacturers, Inc.
REFERENCES
Allen, B. C., Covington, T. R., and Clewell, H. J.
Andersen, M., Sarangapani, R., Gentry, R., Clewell, H., Covington, T., and Frederick, C. B.
Banger, K. K., Lock, E. A., and Reed, C. J.
Banger, K. K., Lock, E. A., and Reed, C. J.
Black, K. A., and Finch, L.
Bogdanffy, M. S., Randall, H. W., and Morgan, K. T.
Bogdanffy, M. S., Sarangapani, R., Kimbell, J. S., Frame, S. R., and Plowchalk, D. R.
Brown, R. P., Delp, M. D., Lindstedt, S. L., Rhomberg, L. R., and Beliles, R. P.
Bush, M. L., Frederick, C. B., Kimbell, J. S., and Ultman, J. S.
Chadha, T. S., Birch, S., and Sackner, M. A.
Chamberlain, M. P., Lock, E. A., Gaskell, B. A., and Reed, C. H.
Custodio, J. B., Palmeira, C. M., Moreno, A. J., and Wallace, K. B.
Frederick, C. B., Bush, M. L., Lomax, L. G., Black, K. A., Finch, L., Kimbell, J. S., Morgan, K. T., Subramaniam, R. P., Morris, J. B., and Ultman, J. S.
Frederick, C. B., Lomax, L. G., Black, K. A., Finch, L., Scribner, H. E., Kimbell, J. S., Morgan, K. T., Subramaniam R. P., and Morris, J. B.
Frederick, C. B., Potter, D. W., Chang-Mateu, M. I., and Andersen, M. E.
Haouzi, D., Lekehal, M., Tinel, M., Vadrot, N., Caussanel, L., Letteron, P., Moreau, A., Feldmann, G., Fau, D., and Pessayre D.
Hellwig, J., Gembardt, C., and Murphy, S. R.
Hoek, J. B., Cahill, A., and Pastorino, J. G.
Licata, A. C., Dekant, W., Smith, C. E., and Borghoff, S. J.
Lomax, L. G., Brown, D. W., and Frederick, C. B.
Mainwaring, G., Foster, J. R., Lund, V., and Green, T.
Miller, R. R., Ayres, J. A., Jersey, G. C., McKenna, M. J.
Miller, R. R., Young, J. T., Kociba, R. J., Keyes, D. G., Bodner, K. M., Calhoun, L. L., and Ayres, J. A.
National Toxicology Program
National Toxicology Program
Potter, D. W., and Tran, T. B.
Saillenfait, E. M., Bonnet, P., Gallissot, F., Protois, J. C., Peltier, A., and Fabries, J. F.
Sweeney, L. M., Gargas, M. L., Strothor, D. E., Kedderis, G. L.
Sweeney, L. M., Tyler, T. R., Kirman, C. R., Corley, R. A., Reitz, R. H., Paustenbach, D. J., Holson, J. F., Whorton, M. D., Thompson, K. M., and Gargas, M. L.
U.S. Environmental Protection Agency (EPA)
Vardeman, S. B.
Vodicka, P., Gut, I., and Frantik, E.
Author notes
*The Sapphire Group, Dayton, Ohio 45431; and †CIIT Centers for Health Research, Research Triangle Park, North Carolina 27709




Comments