-
PDF
- Split View
-
Views
-
Cite
Cite
Robert L. Hamlin, Anusak Kijtawornrat, Bruce W. Keene, David M. Hamlin, QT and RR Intervals in Conscious and Anesthetized Guinea Pigs with Highly Varying RR Intervals and Given QTc-Lengthening Test Articles, Toxicological Sciences, Volume 76, Issue 2, December 2003, Pages 437–442, https://doi.org/10.1093/toxsci/kfg254
Close - Share Icon Share
Abstract
A facile system for obtaining electrocardiograms from conscious animals was used to conduct studies on 12 animals studied both conscious and anesthetized, on 4 conscious animals given vehicle (0.5% methylcellulose) and QT-lengthening test articles, and on 6 animals given test articles thought to not lengthen QTc. In 12 animals whose ECGs were monitored via a bipolar transthoracic ECG, heart rates were slowed with 1.0 mg/kg zatebradine, while they were conscious in their slings, and after being anesthetized with ketamine/xylazine. The following regression equations were obtained relating QT to RR: QT = 44.7 ln RR - 132.9, r2 = 0.7, for conscious animals; QT = 79.4 ln RR - 287.4, r2 = 0.8 for anesthetized animals, with RR intervals varying between 150 and 550 ms. The anesthetic increases QT at all RR intervals (p < 0.001), but does not change the slope of the relationship between QT and RR when compared with the conscious guinea pig. The Fridericia method was best for correcting QT for RR interval in conscious guinea pigs, but the Bazett method was best for correcting in anesthetized animals. QTc lengthened significantly in all conscious guinea pigs given, orally, cisapride, ketoconazole, and sotalol (positive test articles) and did not change with methylcellulose (the vehicle) or with propranolol, verapamil, or enalapril (negative controls). These techniques and relationships demonstrate that this methodology may be useful in exploring torsadogenic effects of novel pharmacological entities.
It is essential to evaluate the potential of a novel pharmacological agent for altering electrophysiology of the heart (Committee for Proprietary Medicinal Products, 1997; International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2002). This includes all electrophysiological properties: chronotropy (measured by heart rate), dromotropy (measured by P, PQ, and QRS durations), QTc, and irritability (spontaneous or provoked ectopia).
Guinea pigs are excellent models for investigating electrophysiology because they are inexpensive, they possess specific ion channels (with the exception of ITO) similar to man (Bünger et al., 1975; Busch et al., 1994; Carmeliet and Zarman, 1979; Khalifa et al., 1999; Roden et al., 1988), they are tractable and clean, they require only small amounts of test article, they are excellent surrogates for predicting the potential of a test article to retard ventricular repolarization in man, and they develop torsade de pointes in response to pharmacological interventions (accepted by J. Pharmacol. Toxicol. Methods.). Because anesthetics are known to alter—either increase or decrease—the sensitivity of a preparation to test articles (Shimizu et al., 1999; Sun et al., 1997), it is highly desirable to study electrophysiology without need for chemical restraint, and furthermore, many forms of physical restraint may inflict discomfort on the animal and produce a sympathetic “storm” which might obfuscate electrophysiological effects of a test article. QT may be corrected for RR interval by a number of methods. Bazett (1920), Fridericia (1920), and Van de Water et al. (1989) are three used commonly. There is inadequate information for the guinea pig to determine which method corrects best for RR interval, and whether or not the best method for the conscious guinea pig would also be the best for the anesthetized animal.
For these reasons, this paper reports a methodology of obtaining electrocardiograms in conscious guinea pigs that requires neither manual restraint nor anesthesia. Because the sum (QT) of the durations of ventricular depolarization (QRS) and repolarization (ST - T) is an important electrocardiographic parameter in predicting a liability of a test article for producing the polymorphic tachycardia, torsade de pointes, values of RR interval and QT obtained from healthy, conscious, but quiet guinea pigs are presented. Because QT is known to lengthen as RR interval lengthens (i.e., as heart rate slows), the mathematical relationship between QT and RR for conscious and anesthetized guinea pigs is presented. Three common methods of correcting QT for RR interval are used to determine which is best for the conscious and which is best for the anesthetized.
Many cardiovascular studies have been performed on conscious guinea pigs (Akita et al., 2002; Gras et al., 1996; Hey et al., 1996); however this study presents a new noninvasive technique to obtain ECGs. In addition, this study is the first to compare different QTc correction factors between conscious and anesthetized guinea pigs.
MATERIALS AND METHODS
This study was approved by the ILACUC of QTest Labs, Inc. A total of 22 guinea pigs were used. All weighed between 350 and 450 grams; half were males and half were females. Six males and six females were used to compare conscious to anesthetized guinea pigs. Ten additional guinea pigs received test articles: four were given test articles known to prolong QTc, and six were given test article known to not change QTc. First they were placed, without chemical interventions, into a comfortable, padded sling, in ventral recumbency. The sling is fitted with copper plates, which sandwich the cranial aspect of the thorax so a bipolar transthoracic electrocardiogram between points rV2 and V2 can be obtained. The electrodes have a hole in the middle so that electrode paste can be injected, without disturbing the guinea pig, through the electrodes and minimize impedance between the electrode and skin. The right and left arm electrodes are attached to the right and left hemithoraces, the electrocardiograph is switched to limb lead I, and a bipolar transthoracic ECG is obtained on a Biopac MP100 Data Acquisition Unit (Biopac Systems, Inc., Santa Barbara, CA). The high pass filter was set at 0.01 Hz, and the low pass filter at 1 kHz, and signals were sampled at 2 kHz. Tracings were obtained for 15 to 60 seconds while the guinea pigs were conscious and quiet. Next, guinea pigs were given, ip, 1.0 mg/kg of zatebradine (Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT), a funny channel blocker, which retards heart rate with no direct effect on any other electrophysiological parameter (Goethals et al., 1993; Hamlin et al., 2003), and tracings were obtained at numerous RR intervals generated in response to zatebradine. This was used to generate curves of QT versus RR interval, since RR interval could be lengthened by zatebradine. RR and QT durations were measured from the ECG at all heart rates, and QT was corrected for RR interval by dividing QT by the cube root of the RR interval. Plots were made of QT versus RR interval, and regression lines with r2 and p values were calculated using Sigma Plot (SPSS Inc., Chicago, IL). QT was corrected for RR interval by dividing QT by the square root (Bazett, 1920) and cube root (Fridericia, 1920) of RR, and by subtracting from QT, 0.087(RR - 1000) (Van de Water et al., 1989). Plots were made to determine which method(s) for the awake and for the anesthetized guinea pigs produced the least dependence of QT on RR. One week later, the entire sequence was repeated, but after the guinea pigs had been anesthetized, ip, with ketamine (33 mg/kg)/xylazine (3.3 mg/kg).
In separate study, four guinea pigs, while conscious and in a sling, were given, orally, 150 mg/kg cisapride, 100 mg/kg ketoconazole, and 100 mg/kg sotalol, in 0.5 ml of 0.5% methylcellulose (the vehicle). Six additional guinea pigs (negative controls) were given, orally, propranolol (10 mg/kg), verapamil (2.5 mg/kg), and enalapril (5 mg/kg) in 0.5 ml of methylcellulose. All doses were selected using information from the literature (Cushman et al., 1989; Gras et al., 1996; Hey et al., 1996; Kii et al., 2001; Levy, 1976; Meuldermans et al., 1998; Saitoh et al., 2002; Yaoita et al., 2002). There was no randomization of exposure to test articles, because at least 3 days (> 8 half lives) occurred between dosing. Bipolar transthoracic ECGs were obtained before dosing, and every 30 minutes postdosing for 2 hours. All test articles are known to achieve Tmax before 2 hours. Recordings were made for between 15 and 30 seconds, which included >70 beats. Measurements were made of at least 10 consecutive cardiac cycles, and the average was used. Plots of mean values versus time were made, differences between each test article and vehicle were calculated, and differences of significance between each test article and vehicle were sought by a one-way, ANOVA with repeated measures design on time. When indicated by a significant F statistic, specific means were compared by the Tukey post-hoc test.
RESULTS
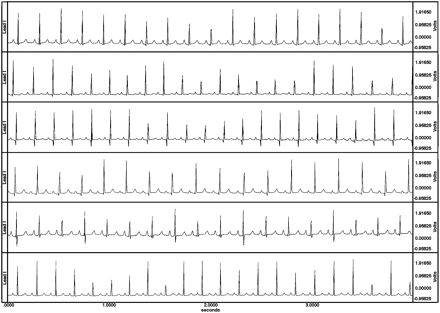
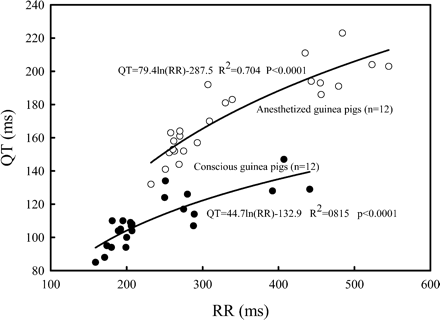
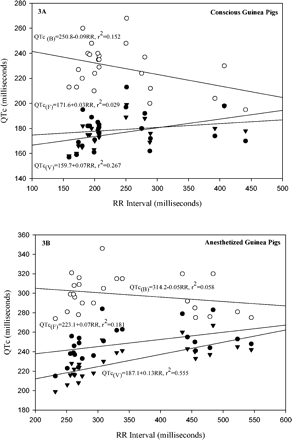
Most guinea pigs fell asleep or were extremely quiet within 1 minute after being placed in the sling; they awakened briefly when the zatebradine was given, but then they either fell asleep or remained quiet until being removed from the sling. Electrocardiograms of excellent quality for interpretation were obtained from all conscious (Fig. 1) and anesthetized guinea pigs. Plots of QT duration versus RR duration for conscious and for anesthetized guinea pigs are shown (Fig. 2). The curvilinear relationships are expressed by the equations; both r2 and p values are shown. The r2 demonstrates that more than 70% of the variability of QT for the awake guinea pigs and more than 80% of r2 for the anesthetized guinea pigs are determined by RR interval, and that the relationships are highly significant (p < 0.001) for both. It can be observed that the anesthetic displaces the curve upward (i.e., longer QTs for anesthetized than for conscious) compared to the curve from the naturally sleeping or quiet guinea pigs, but that the slope does not change significantly. Plots (Figs 3A and 3B) of QTc, using all three methods of correction for RR interval, were made against RR. Slope for the equations regressing QTc with RR intervals was smallest for the Fridericia (r2 = 0.029) method for the awake guinea pigs, but was smallest for the Bazett method (r2 = 0.058) for the anesthetized guinea pigs.
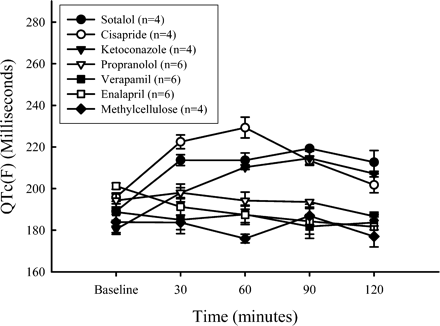
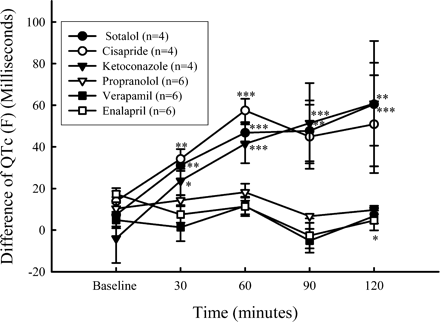
QTc(F) lengthened in all guinea pigs given any of the test articles known to lengthen QTc and failed to lengthen when given methylcellulose or test articles thought to not lengthen QTc. Figure 4 shows the mean values and SEM of QTc, both before and after dosing, and Figure 5 shows the differences between QTc for each test article and methylcellulose. The levels of significance in the difference are labeled by asterisks. None of the guinea pigs developed arrhythmias coincident with QT lengthening.
DISCUSSION
The purposes of this study, to demonstrate that ECGs of high quality could be obtained, to compare QT intervals from conscious and anesthetized guinea pigs, and to establish a correction for QT based upon RR interval, were all achieved. Furthermore, it was demonstrated that QTc lengthening occurred in response to all three test articles thought to lengthen QTc, and failed to lengthen in response to all three test articles thought to not lengthen QTc. This study demonstrates that a single bipolar, transthoracic ECG, from which RR and QT may be measured easily, can be obtained from guinea pigs placed in a comfortable sling, that a relationship between QT and RR can be modeled by a logarithmic relationship, and that anesthesia with ketamine/xylazine displaces upward the curvilinear relationship.
The study reports that a single lead is adequate for measuring intervals important to identify a liability of a test article for lengthening ventricular repolarization. If the guinea pig heart may be modeled as an equivalent dipole as for the dog and man (Hamlin et al., 1968), three mutually orthogonal leads should provide over 85% of the electrocardiographic information available from a greater number of leads, but a single lead—as used in this study—provides the dominant portion of dipolar information. There is no evidence, from toxicological studies or from studies in safety pharmacology, that test articles force the model to become more than dipolar; therefore information from the transthoracic bipolar ECG reported here is adequate for identifying the most common manifestations of toxicity (e.g., changes in heart rate, production of arrhythmia, lengthening of QT). Also, a single bipolar transthoracic lead with electrodes close to the heart produces high voltages and is less likely to produce ECGs that are not obfuscated by 60 Hz or muscle tremor artifacts.
This study shows that the method of Fridericia for correcting QT for RR interval of conscious guinea pigs is the best, but that the method of Bazett is best for the anesthetized guinea pigs, consistent with the observations of Hayes et al. (1994) and De Clerck et al. (2002). The “best” relationship is defined as the one with a slope for the regression equation closest to 0. Of course there are many other methods for correcting, but with more than 70% of the variability of QT depending upon RR, QTcs calculated in this study are useful for identifying a QTc liability independent of heart rate.
Ketamine/xylazine can be used as a “universal” anesthetic (Flecknell, 1996), since all animals can be safely and effectively anesthetized with it. The slope of the relationship between QT and RR is not changed by the anesthetic, but the curve is displaced upward. With a greater number of guinea pigs, it is possible that the slopes between conscious and anesthetized may differ. There is no data available to demonstrate if either the displacement or slope would hold true for other anesthetic regimen. Nonetheless, the fact that ketamine/xylazine produced a significant displacement of the QT versus RR relationship establishes the possibility that other anesthetics may affect the displacement or the slope even more than ketamine/xylazine. In addition, anesthetics may alter renal and hepatic blood flows and rates of metabolism and/or excretion. Finally, all anesthetics—even morphine/chloralose—affect autonomic tone, which may alter electrophysiological effects of test articles. Thus if at all possible, conducting studies on conscious subjects should be preferred.
This study also showed that this model is sensitive for detecting lengthening of QTc for three compounds known to lengthen QTc, while showing no lengthening due to vehicle or to other test articles known not to lengthen QTc. Blood levels of the test articles were not measured, but the doses of test articles given were close to those given orally to other experimental animals (guinea pig and rat) (Cushman et al., 1989; Gras et al., 1996; Hey et al., 1996; Kii et al., 2001; Levy, 1976; Meuldermans et al., 1998; Saitoh et al., 2002; Yaoita et al., 2002).
The greatest features of this preparation are the speed, ease, and lack of psychological stimulation with which ECGs may be obtained in extremely quiet or naturally sleeping guinea pigs, a species whose heart possesses great similarities to man in terms of specific ion channels (except for ITO). This study adds credibility to the use of guinea pigs in toxicology and safety pharmacology and provides data against which future studies may be compared; in particular the study showed that QTc prolongation can be identified in conscious guinea pigs dosed orally. Because the guinea pigs became extremely quiet or asleep within 60 seconds of placing them in the sling, this method permits following the time-course of changes in QTc without need for chronic instrumentation. Because of how quickly the guinea pigs fall asleep once in the sling, it would be possible to measure drug effects every 1 minute, without those effects being obfuscated by psychological influences. This would be nearly impossible in many other species, which either literally never “settle down” or require a prolonged period to obfuscate psychological factors.
Bipolar, transthoracic ECGs from 6 of the 12 conscious guinea pigs. Notice the absence of baseline artifacts, the ease of identification of the onset of QRS and the end of T, and the fluctuations in height of R waves as the guinea pigs breath.
Plots of QT versus RR interval for all guinea pigs, both conscious (closed circle) and anesthetized (opened circle). Notice that the RR intervals varied from approximately 150 ms (a heart rate of 400/minute) to 550 ms (a heart rate of 109/minute). Each data point is an average of 10 consecutive cardiac cycles from a different guinea pig.
Plots of QTc corrected by three methods versus RR interval for all guinea pigs, either (A) conscious or (B) anesthetized. Notice that the Bazett (opened circle) correction is best for the anesthetized guinea pigs, but that the Fridericia (closed circle) correction is best for the conscious guinea pigs, while the Van de Water formula (closed triangle) failed to remove the influence of heart rate in both conscious and anesthetized guinea pigs. Each data point is an average of 10 consecutive cardiac cycles from a different guinea pig.
Plots of means and SEM of QTc(F) versus time after dosing of three test articles known to lengthen QTc(F), three test articles known not to lengthen QTc(F), and for the methylcellulose vehicle. Each data point is the average of 10 consecutive cardiac cycles. Notice the lengthening of QTc(F) for the three test article but not for the vehicle. Each mean is the mean of four guinea pigs receiving positive test articles, six guinea pigs receiving negative test articles, and four guinea pigs receiving vehicle (methylcellulose). Doses of test articles were in mg/kg: sotalol (100), cisapride (150), ketoconazole (100), propranolol (10), verapamil (2.5), enalapril (5), methylcellulose (0.5%).
Plots of means and SEM of the difference between QTc (in ms) for each test article and the vehicle. Each data point is the average of 10 consecutive cardiac cycles. An asterisk indicates when a difference changed with statistical significance from baseline. One asterisk indicates a p < 0.05, two asterisks indicate a p < 0.01, and three asterisks indicate a p < 0.001. Each mean is the mean of four guinea pigs receiving positive test articles, and six guinea pigs receiving negative test articles. Doses of test articles were in mg/kg: sotalol (100), cisapride (150), ketoconazole (100), propranolol (10), verapamil (2.5), enalapril (5).
To whom correspondence should be address at QTest Labs, Inc., 6456 Fiesta Drive, Columbus, OH 43235. Fax: (614) 760-0900. E-mail:rhamlin@qtestlabs.com.
COI: This study was conducted in a company, QTest Labs, for which the senior author is a paid consultant, and the last author is a partial owner.
REFERENCES
Akita, M., Ishii, K., Kuwahara, M., and Tsubone, H. (
Bünger, R., Haddy, F., Querengässer, A., and Gerlach, E. (
Busch, A. E., Malloy, K., Groh, W. J., Varnum, M. D., Adelman, J. P., and Maylie, J. (
Carmeliet, E., and Zarman, M. Y. (
Committee for Proprietary Medicinal Products. (
Cushman, D. W., Wang, F. L., Fung, W. C., Grover, G. J., Harvey, C. M., Scalese, R. J., Mitch, S. L., and DeForrest, J. M. (
De Clerck, F., Van de Water, A., D’Aubioul, J., Lu, H. R., van Rossem, K., Hermans, A., and Van Ammel, K. (
Flecknell, P. A. (
Fridericia, L. S. (
Goethals, M., Raes, A., Van Bogaret, P. P. (
Gras, J., Llenas, J., Palacios, J. M., and Roberts, D. J. (
Hamlin, R. L., Nakayama, T., Nakayama, H., Carnes, C. A. (
Hamlin, R. L., Pipers, F. S., Smith, C. R. (
Hayes, E., Pugsley, M. K., Penz, W. P., Adaikan, G., and Walker, M. J. (
Hey, J. A., del Prado, M., Sherwood, J., Kreutner, W., and Egan, R. W. (
International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. (
Khalifa, M., Drolet, B., Daleau, P., Lefez, C., Gilbert, M., Plante, S., O’Hara, G. E., Gleeton, O., Hamelin, B. A., and Turgeon, J. (
Kii, Y., Nakatsuji, K., Nose, I., Yabuuchi, M. Yasuyuki, Y., and Ito, T. (
Levy, J. V. (
Meuldermans, W., Hendrickx, J., Lauwers, W., Hurkmans, R., Mostmans, E., Swysen, E., Bracke, J., Knaeps, A., and Heykants, J. (
Roden, D. M., Bennett, P. B., Snyders, D. J., Balser, J. R., and Hondeghem, L. M. (
Saitoh, M., Sugiyama, A., Nakazawa, T., and Hashimoto, K. (
Shimizu, W., McMahon, B., and Antzelevitch, C. (
Sun, Z. Q., Eddlestone, G. T., and Antzelevitch, C. (
Van de Water, A., Verheyen, J., Xhonneux, R., and Reneman, R. S. (




Comments